Background. Clonal hematopoiesis (CH) prevalence increases with age. Elderly (>70 years) healthy individuals have a 10% incidence of CH. Cancer cohorts are at increased risk of harboring CH mutations (~25%). Specific tumor types such as thyroid, ovarian, and bladder, have previously been shown to be at the highest risk of CH due to increased exposure to radionuclide and chemo-radiotherapies. Radionuclide therapies in particular are associated with a high incidence of subsequent myeloid disorders. Exposure to peptide receptor radionuclide therapy (PRRT, Lu 177 dotatate) leads to an increased risk (~2-5%) of therapy related myeloid neoplasms (MNs). It is not known if baseline CH architecture of neuroendocrine tumors (NETs) contributes to the increased risk or the protracted course of NETs or exposure to leukemogenic therapies. With a broader view to understand emergence of therapy induced myeloid neoplasms in various malignancies, we aimed to characterize baseline rates of CH in patients (pts) with NETs and assess clonal evolution in serial blood samples procured prior to and after exposure to PRRT.
Methods. The NET Biobank housed at Roswell Park Comprehensive Cancer Center contains banked serial samples for pts receiving PRRT since its FDA approval in 2018. In this retrospective analysis, we identified pre-PRRT blood samples from 13 pts with NET treated at our institute from 2018-19. Serial samples (post PRRT exposure) were available for 6 of 13 pts. Genomic DNA collected from pts before and after PRRT treatment initiation was analyzed for CH mutations using a custom panel targeting 93 genes. A VAF cut off of 1% was used to define putative CH mutations. Relationships among clinical, laboratory and mutational variables were examined using chi-square test, at a significance level of 0.05.
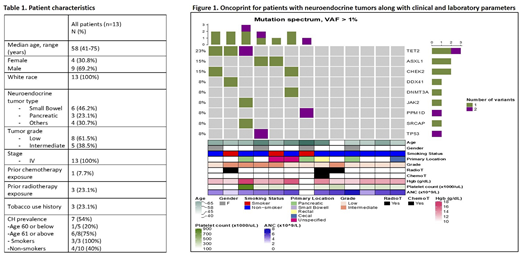
Results. Pt characteristics (n=13) are shown in Table 1. Median age was 58 years. 70% were men. Only 1/13 pt had prior chemotherapy exposure, and 3/13 had prior RT exposure. The primary location of NETs was small bowel (46%) followed by pancreas (23%) and others (cecal, rectal or unknown - 31%). All pts had stage IV disease. Over half (54%) harbored CH mutations, despite their relatively young age. This prevalence is much higher than previously reported CH prevalence of 25.1% in pts with other solid tumors.1TET2 (25%) was the most commonly mutated gene, followed by ASXL1 (12.5%), CHEK (12.5%), PPM1D (12.5%) and TP53 (12.5%) which together accounted for 50% of all mutations. Other mutations (DNMT3A, JAK2, DDX41, SRCAP) were less common (Figure 1). DNMT3A mutations (usually most prevalent mutation in cancer cohorts) were uncommon in this cohort and did not occur in the R882 AML/MDS hotspot, previously described in CH. Frameshift mutations and truncations comprised 53.3% of all mutations, and these mutations had higher mean VAFs (6.14%) than single nucleotide variants (1.93%; p = 0.001). This may suggest that frameshift mutations and truncations provide survival advantage to the affected clone. Mutations were more common in smokers than in non-smokers (100% vs 40%, p = 0.067). As expected, mutations were more common in individuals age 61 and above (75% harbored 1 or more mutation) vs only 20% mutation occurrence was noted in age 60 or below (p=0.05).
Conclusion. CH with a distinct mutation profile occurs in NET patients, and in higher prevalence (54%) than observed in other solid tumors (25%). High baseline prevalence of putative CH mutations in NET patients may be an important contributor to heightened risk of MN development after PRRT exposure. Ongoing serial sample evaluation will provide further insights into clonal evolution of the above detected CH mutations after exposure to PRRT. The results regarding clonal evolution may have implications in predicting risk of MN associated with PRRT therapy and influence treatment selection in pts planned for PRRT.
References
Coombs CC, Zehir A, Devlin SM, et al. Therapy-Related Clonal Hematopoiesis in Patients with Non-hematologic Cancers Is Common and Associated with Adverse Clinical Outcomes. Cell Stem Cell. 2017;21(3):374-382.e374.
Acknowledgement: Data and samples for this study were provided by the Data Bank and BioRepository (DBBR), which is funded by the National Cancer Institute (P30 CA016056) and is a Roswell Park Cancer Institute Cancer Center Support Grant shared resource.
Iyer:Advanced Accelerator Applications: Consultancy. Guzman:SeqRx: Honoraria; Cellectis: Research Funding. Wang:Abbvie: Consultancy; Jazz Pharmaceuticals: Consultancy; Genentech: Consultancy; Stemline: Speakers Bureau; PTC Therapeutics: Consultancy; Astellas: Consultancy; Macrogenics: Consultancy; Pfizer: Speakers Bureau; Bristol Meyers Squibb (Celgene): Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.